Unsubscribe
To unsubscribe, please enter your e-mail address below or send us an e-mail at unsubscribeeu@blueprintmedicines.com
Please enter a valid e-mail address
Regular assessment of symptom burden and disease progression is essential for the timely and appropriate management of SM. Treatment options for both ISM and Advanced SM now extend beyond supportive care – ranging from symptomatic therapies to available targeted therapies.1
Visible and non-visible symptoms contribute to the true burden of ISM. Non-visible manifestations of ISM that affect functioning such as brain fog and fatigue are frequently experienced by patients but under-reported.2 Objective markers such as serum tryptase and the presence of visible symptoms are not well correlated with impact on everyday functioning in ISM.2,3
Routine assessment and proactive dialogue about patient quality of life (QoL) help to reveal the ongoing impact of ISM on everyday function4
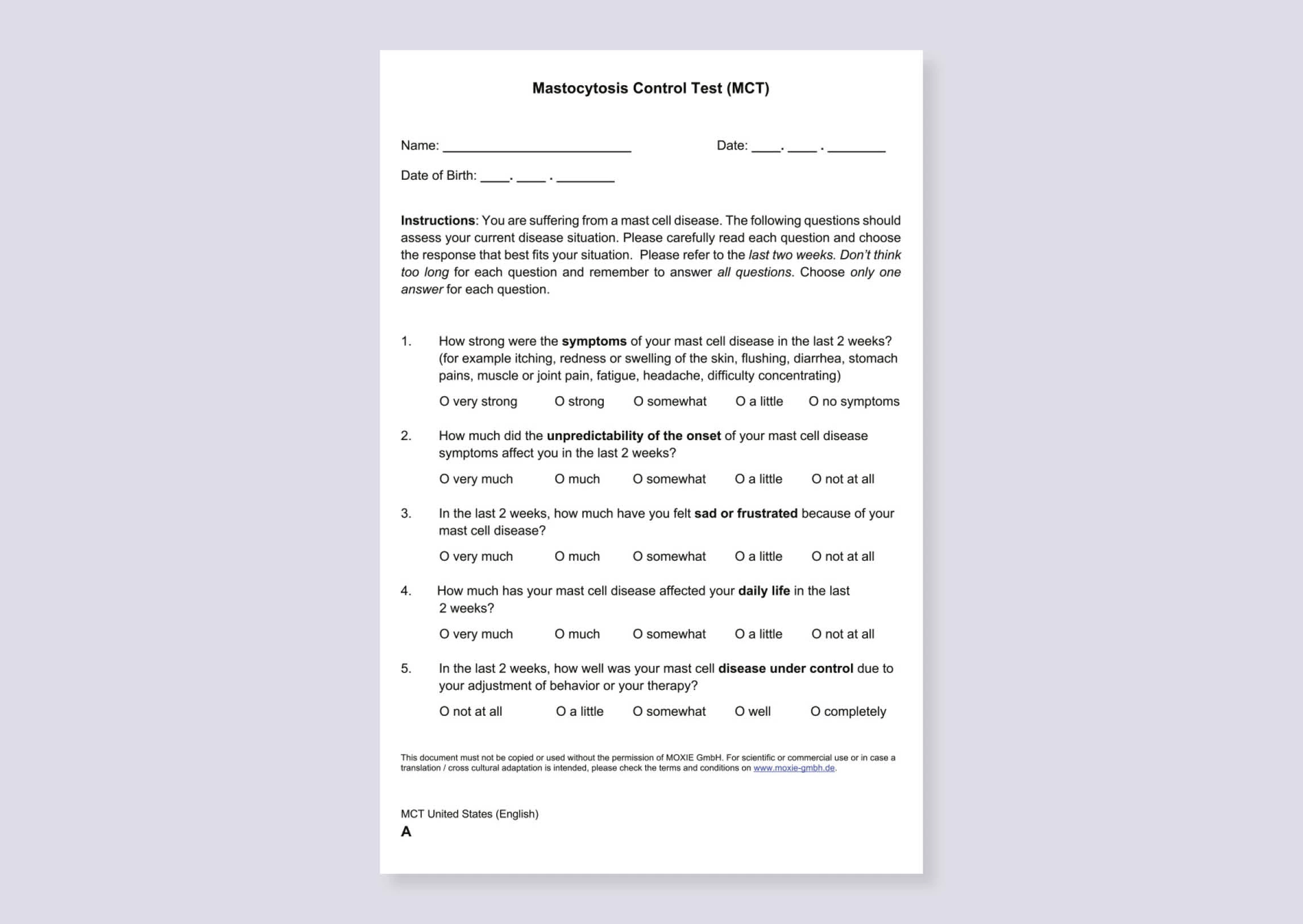
The Mastocytosis Control Test (MCT) is a validated and standardised patient-reported outcome tool for assessing and monitoring disease control in ISM in clinical practice4
"We try to follow our ISM patients periodically and monitor, in particular, how the symptoms and signs change over time. It is very important to talk to the patients and ask for the onset of new symptoms or any changes to symptoms they reported at previous visits."Dr Cristina Papayannidis
Revisit the burden of disease to understand the impact on patients' QoL
KIT D816V VAF (variant allele frequency) and S/A/R (SRSF2, ASXL1, RUNX1) gene panels help reveal prognostic information in Advanced SM, independent of the associated haematological neoplasms5–7
KIT D816V VAF can be a useful prognostic marker for assessing disease progression in Advanced SM5,6
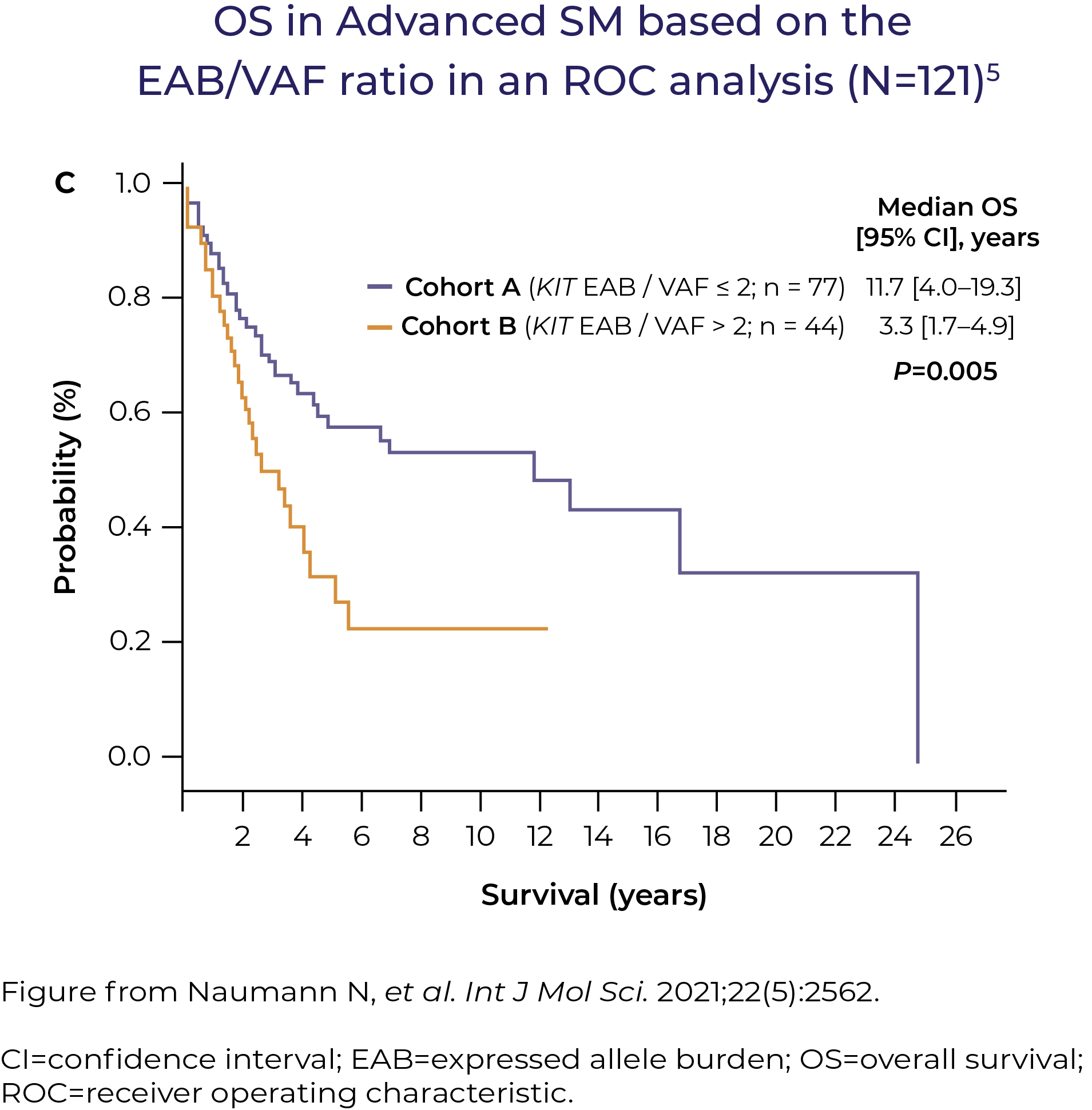
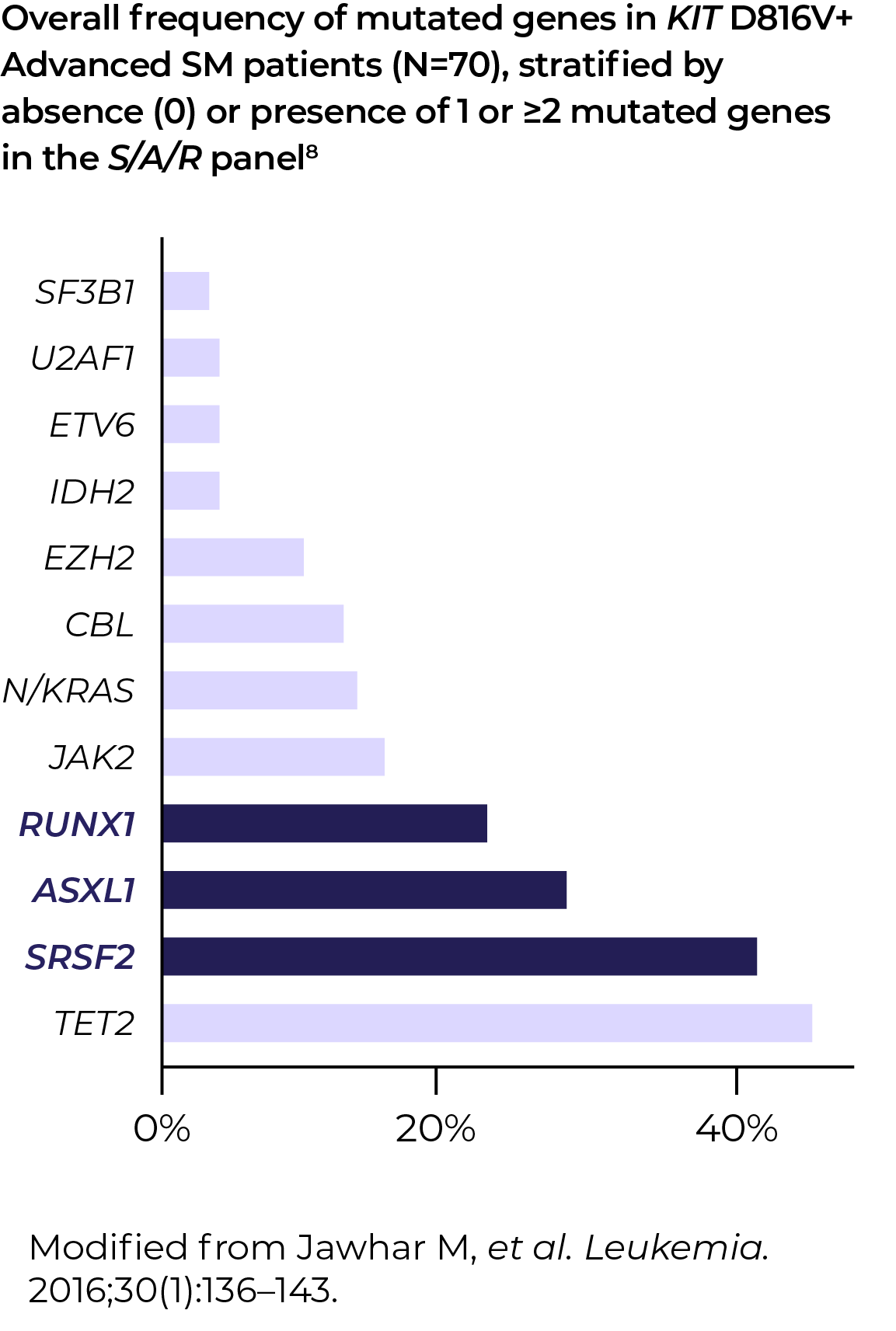
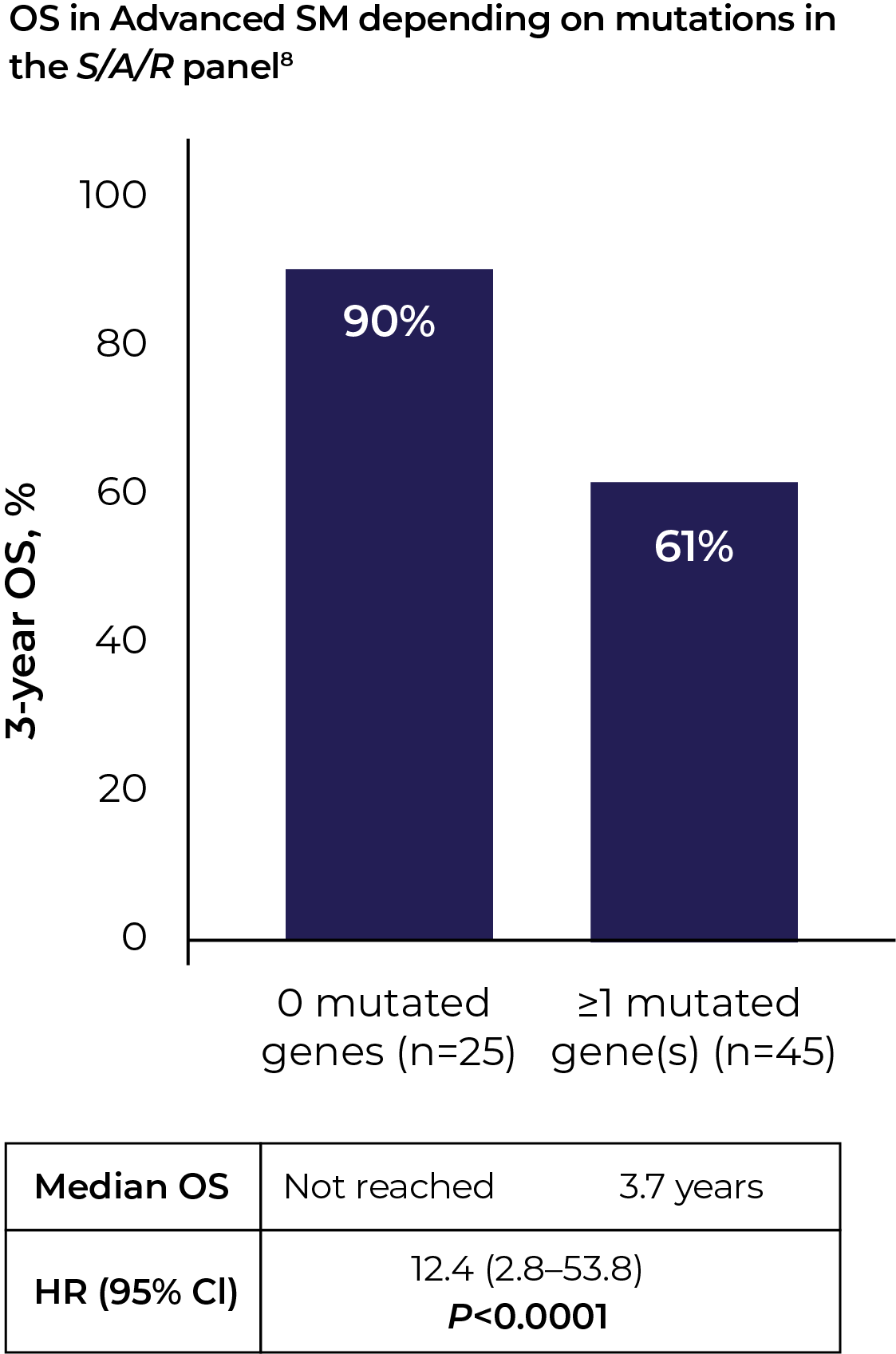
"If I were seeing an Advanced SM patient, and even if it was picked up on NGS, I would still do digital droplet PCR for two reasons: confirmation, and to get the VAF. Moving forward, I think we're going to need to look at both the value and the allele fraction – first, to assess disease burden, and second, to evaluate treatment effectiveness."Dr Deepti Radia
Read our publication summaries to learn more
Want to learn more about Systemic Mastocytosis?
Visit our Resources Page for more information.
Take me there!